Summary
In 2019 the European Commission (EC) requested that the European Food Standards Agency (EFSA) revise their original 2013 Guidance document on risk assessment of plant protection products on bees (Apis mellifera, Bombus spp and solitary bees). Following the publication of the new revised draft document and a public consultation process in 2022, EFSA have now published the final document.
The revised guidance is available here.
Background
The 2013 Bee Guidance Document from EFSA was never fully endorsed by the majority of Member States in the Standing Committee on Plants, Animals, Food and Feed despite attempts over several years.
In March 2019 the EC mandated EFSA to review the 2013 Bee Guidance Document, taking scientific knowledge and developments that had emerged since the publication of the original document into account.
The terms of reference of the mandate were to:
- Consider feedback from stakeholders
- Review bee background mortality
- Review crop attractiveness
- Review Risk Assessment methods
- Review requirements for higher tier testing
- Consider Specific Protection Goals (SPGs) from Risk Managers
Scope
- Provide guidance in the context of the evaluation of PPPs and their active substances under Regulation (EC) 1107/2009
- The focus is on direct effects; indirect effects are out of scope
- The focus is on chemicals, the Guidance does not cover the risk assessment for microbial biopesticides
EFSA Milestones
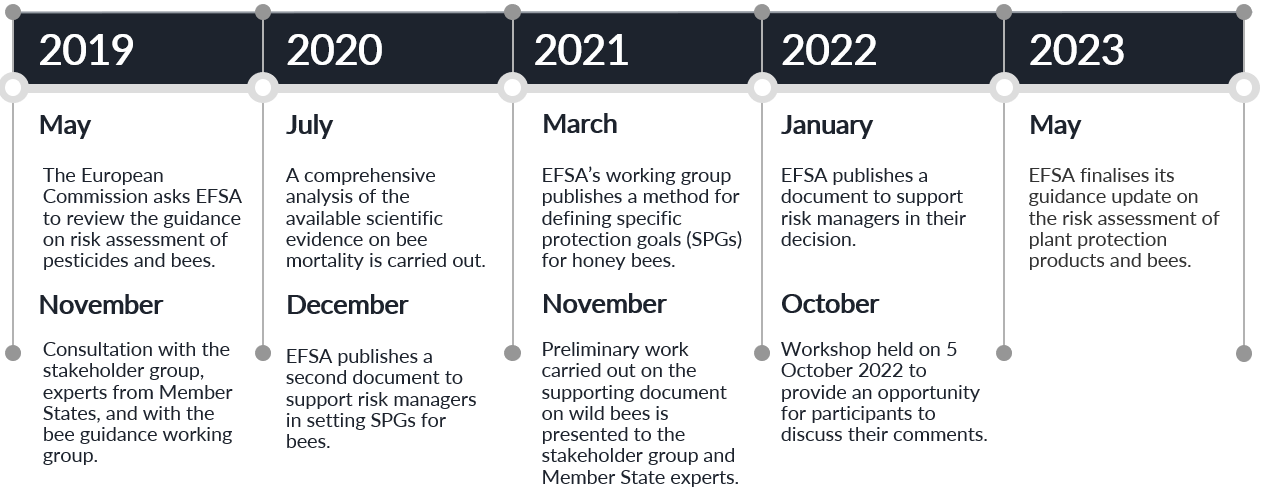

Click here to view all milestone
Main Changes
- New SPG (Specific Protection Goal) - increased from 7% to 10%
- Exposure estimations have been amended updated; plus new parameters included
- Detailed guidance for Tier 2 options
- Hazard characterization reconsidered
- Extrapolation between species amended to use Toxicity Extrapolation Factors (Tef) where a surrogate endpoint is used
- Risk Assessment method reconsidered
- Statistical approaches have been updated
- Introduction of Time Related Toxicity (TRT, previously accumulative toxicity)
- Exposure via water routes (e.g. guttation, surface water) removed
- Considerations for sublethal effects, metabolites and many more issues update
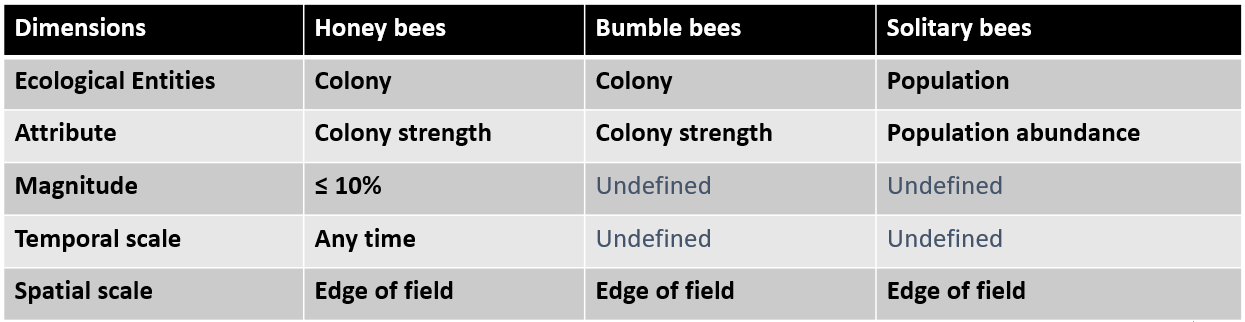
SPG agreed by Risk Managers

Pollinator Ecotoxicology
From standard laboratory studies to the more complex, bespoke higher tier studies designed to address specific risk assessment needs, our experts at Fera are perfectly placed to meet your data requirements, and to help our partners develop products that are safer for bees and other pollinators.
To help refine your risk assessment, Fera can undertake a range of higher tier cage, tunnel, or semi-field studies. Our capabilities include the Oomen Brood Feeding Study and the Honey Bee Brood Test under semi-field conditions (OECD GD 75).
If you need guidance on your pollinator study needs or are concerned about the impacts to your bee risk assessments due to the changes, our regulatory specialists are on hand to chat with you.




